- Review
- Open access
- Published:
Emerging roles of liquid-liquid phase separation in liver innate immunity
Cell Communication and Signaling volume 22, Article number: 430 (2024)
Abstract
Biomolecular condensates formed by liquid-liquid phase separation (LLPS) have become an extensive mechanism of macromolecular metabolism and biochemical reactions in cells. Large molecules like proteins and nucleic acids will spontaneously aggregate and assemble into droplet-like structures driven by LLPS when the physical and chemical properties of cells are altered. LLPS provides a mature molecular platform for innate immune response, which tightly regulates key signaling in liver immune response spatially and physically, including DNA and RNA sensing pathways, inflammasome activation, and autophagy. Take this, LLPS plays a promoting or protecting role in a range of liver diseases, such as viral hepatitis, non-alcoholic fatty liver disease, liver fibrosis, hepatic ischemia-reperfusion injury, autoimmune liver disease, and liver cancer. This review systematically describes the whole landscape of LLPS in liver innate immunity. It will help us to guide a better-personalized approach to LLPS-targeted immunotherapy for liver diseases.
Introduction
Cells normally perform biological functions depending on complex spatiotemporal regulation, where various biochemical reactions happen effectively and in an orderly manner in a specific time and area [1]. The membraneless organelles are subcellular compartments with high local density driven by LLPS with biological macromolecules such as proteins and nucleic acids, which are also known as biomolecular condensates [2]. LLPS plays an important role in the normal activities of life, including gene transcription and translation, chromatin organization, signal transduction, autophagy, DNA repair, cell bonding, and immune response [3]. However, despite cells have developed a variety of mechanisms to ensure the coordinated state of LLPS, the abnormal LLPS will contribute to diseases in many tissues and organs, including nerves, kidneys, lungs, intestines, and others [4,5,6].
Recently, LLPS has moved beyond its basic biophysical profile to be significantly correlated with immune status [7]. Innate immunity is the body’s comprehensive defense against the invasion of foreign pathogens, which uses a series of germline-encoded pattern recognition receptors to detect conserved microbial structures, including viruses, bacterial components, and endogenous damage factors released by cells. LLPS works through the dynamic recombination of molecules or isolates pathogenic agents to exactly start and amplitude immune signaling, which brings a new perspective to our understanding of immune-related diseases [8].
As a central immune organ, the liver is densely populated with Kupffer cells (KCs) and lymphocytes, such as natural killer (NK) cells, T cells, and B cells [9]. KCs are the resident macrophages in the liver, endowed with robust capabilities of antigen recognition, presentation, and phagocytic. Mitochondrial fission generates intracellular high calcium ions, inhibiting the formation of WIP/WASP droplets, KCs phosphorylate WIP and enhance the phagocytosis of tumor cells [10]. Vitro recombinant experiments have demonstrated that the intracellular domain of the CD3ε subunit of T cell receptor can undergo LLPS with Lck, which facilitates rapid T cell activation [11]. There may also be unreported LLPS droplets involved in the interaction between NK cells and their ligands during the apoptosis and ADCC of target cells.
Immune cells express multiple pattern recognition receptors (PRRs) to recognize pathogens [12]. Thus, transcription factors such as IRF3, IRF7, and NF-κB will transfer into the nucleus to activate the genes’ transcription, and then release interferon (IFN), and cytokines [13]. The dynamic changes in liver immunity caused by these molecules determine the development and evolution of liver diseases.
Therefore, we summarized the role of LLPS in cyclic GMP-AMP synthase (cGAS)- stimulator of interferon genes (STING), retinoic acid-inducible gene I protein (RIG-I)- mitochondrial antiviral signaling protein (MAVS), inflammasome pathways, and autophagy, and sorted out liver diseases based on this framework. Finally, we introduce LLPS in liver cancer and look forward to the progress of anti-tumor therapies targeting it. In short, this review provides a reliable basis for understanding of LLPS in liver innate immunity.
LLPS at a glance
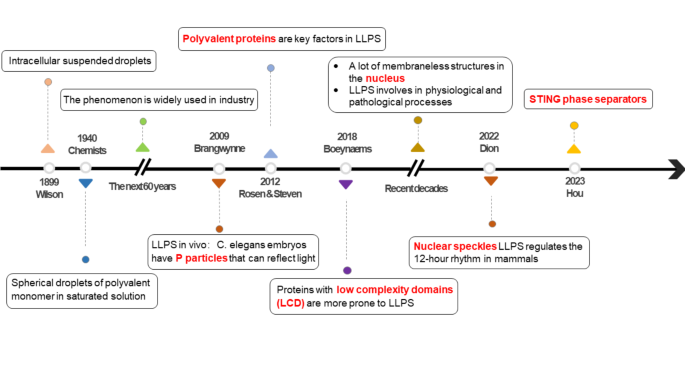
The research history of LLPS
Brangwynne was the first to discover the liquid reflecting light in C. elegans embryo, also known as, P particles, which exhibit liquid-like behavior, such as dissolution, condensation, and attachment, they indicate intermolecular interaction within the cytoplasm [14]. In 2012, cryo-electron microscopy demonstrated that proteins rich in repeated charged amino acid residues and with multiple folded domains (such as the SH3 domain) contribute to LLPS [15]. In recent years, it has been approved that proteins with low complexity domain (LCD) were easier to drive LLPS through weak interactions between their amino acid residues. For example, fused fused in sarcoma (FUS) relies on tyrosine or phenylalanine residues to achieve LLPS [16] (Fig. 1).
Biomolecular Condensates in eukaryotic cells. A) Schematic of the numerous condensates in the nucleus, cytoplasm, and membranes, such as stress granule, Cajal body, P-body, nuclear speckles, et al. B) Internal structure model of condensates. For example, the stress granule is driven by liquid-liquid phase separation. It is composed of proteins and RNA from the dispersed state into a highly aggregated droplet-like structure, which displays liquid-like properties
With the development of bioinformatics algorithms, more specific structures and functions of LLPS have been reported. Significantly, LLPS-regulated membraneless structures are abundant in mammalian cells [17, 18]. Nucleolus is an insoluble droplet composed of RNA and protein, which displays liquid-like viscous relaxation and effective surface tension [19]. As a self-protection mechanism for cell growth and survival, when eukaryotes face external stress, mRNA and protein are packaged into ribonucleoprotein particles, namely stress granules (SGs). When specific conditions are activated, translation can be restarted [20]. Intervention in the assembly and depolymerization of biomolecular aggregates such as SGs provide new ideas for the diagnosis of diseases (Fig. 2).
Formation conditions
Polyvalent molecules in biomolecular condensates naturally tend to aggregate into a large polymer in a heterogeneous state, similar to the layering and isolation of two phases in vinaigrettes, which reduce the solubility, thus contributing to LLPS [21]. LLPS is driven when the macromolecular components in high concentrations, exhibit a stronger affinity for each other compared to cytoplasmic molecules, and different phases are equal in chemical potentials [21, 22]. Eventually, surface tension leads to the spherical structure.
Importantly, it has been indicated that the LCD, intrinsically disordered region (IDR), and weak polyvalent interactions of the protein serve as triggers for LLPS [1]. Weak interactions, such as π-π interactions [23], cation-π interactions [24], electrostatic interactions [25], and transient cross-β contacts [26]. LLPS exhibits a remarkably dynamic and rapid ability to interact with the external environment in biomolecular condensates. Indeed, any perturbation that influences protein structures or intermolecular contacts can disturb LLPS behavior.
Exogenous infections can also evoke LLPS. Viral infection can lead to protein aggregation, which may be due to the exogenous nucleic acid molecules stimulating and attracting a substantial quantity of antiviral immune molecules. For instance, heat shock proteins are frequently integrated into virions via LLPS to facilitate the assembly and enhance the proteins’ stability [27].
Regulation methods
Physicochemical property and post-translational modification (PTM) regulate LLPS
The alteration of physical and chemical properties is the fundamental element for LLPS, such as component concentration, temperature, pH, valency, salt concentration, and PTM of proteins, like phosphorylation, methylation, ubiquitination, acetylation, etc. [28, 29]. Proteomic analysis has successfully identified a total of 14 serine or threonine phosphorylation sites on LCR [26, 30]. Sang has shown that the C-terminal fragment of MAPK3 will accelerate the serine site phosphorylation of substrate ELK1 after recruitment. This finding suggests that the condensate itself can modulate the new links in the LLPS network by increasing the phosphorylation rate [31]. Furthermore, the methylation of arginine residues can enhance the hydrophobic properties of RNA-binding proteins, like FUS, consequently impeding LLPS.
PTMs are closely associated with a variety of diseases, including cardiovascular disorders, liver diseases, and neurological disorders [32]. They play a crucial role in coordinating gene expression, metabolic reprogramming, and immune recognition. Deviations in these modification levels can lead to genomic abnormalities, cellular metabolic disruptions, and immune evasion [33]. Consequently, PTMs influence LLPS by changing the charge distribution and hydrophobicity of key protein IDRs, which encompasses both up-regulation and down-regulation, further impacting disease progression.
Certain core molecules regulate LLPS
LLPS is regulated by several “switch” molecules including GAP SH3 Binding Protein 1 (G3BP1), G3BP2, ATP, etc. Cells can produce SGs to protect themselves from damage under stress [34]. As a structural composition of SGs, G3BP1 possesses a nuclear transport 2-like domain, an inherently disordered region, and an RNA binding domain that contains RNA-recognized and RGG motifs. Under no-stress conditions, G3BP1 is in a closed conformation [35]. Under external stimulation, an elevated RNA in the cell induces RNA-dependent LLPS, causing a change into an open G3BP1 conformation. During this, the RGG domain binds to RNA, and phosphorylation occurs at S149, which promotes LLPS [36]. Further studies have revealed that the interaction between Caprin1 and G3BP1 with NTF2 can enhance the formation of SGs. Conversely, USP10 acts as a negative regulator of SGs assembly by obstructing the binding site of NTF2 [37]. In addition, it has been confirmed that ATP can also promote LLPS, for example, free nucleotides promote the formation of droplets in nucleosomes within H1 chromatin. This finding highlights the potential role of energy molecules in LLPS regulation [38]. Recently, 1,518 endogenous phase-separating proteins have yet to be discovered in a quantitative and high-throughput manner [39]. Therefore, we look forward to more factors involved in LLPS assembly to develop precise molecular targets.
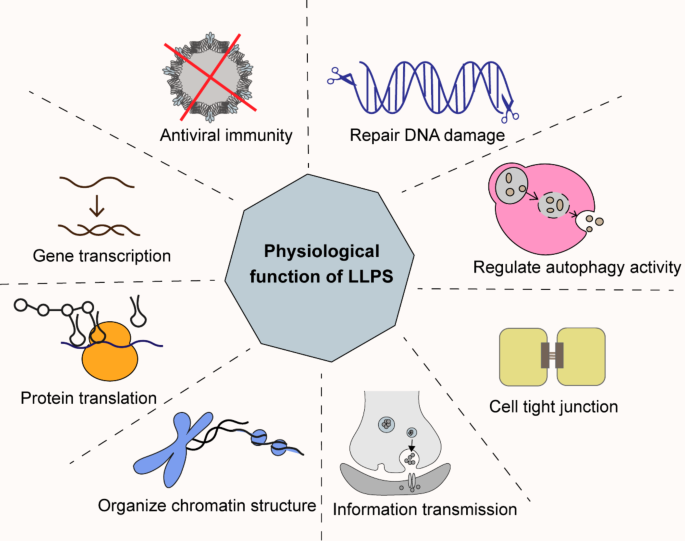
The physiological function of LLPS
Gene transcription and protein translation
The core proteins involved in transcriptional activation are formed through LLPS aggregation, including RNA polymerase II, transcription factors, coactivators, and elongation factors [40]. During transcription initiation, LLPS recruits Pol II to gather at active gene sites and enhances concentration between DNA and transcription factors to form the transcription initiation complex [41]. During transcription elongation, the C-terminal domain of Pol II is phosphorylated by the transcription elongation factor P-TEFb, and aggregates with other proteins, ultimately leading to complete the transcription [30]. Interestingly, super-enhancers are formed by transcription factors and co-activators through LLPS, which may provide insights into the excessive activation of tumor genes [42] (Fig. 3).
Translating RNA into proteins also requires LLPS. Recent studies have provided evidence that ATXN2 facilitates the translation of the circadian rhythm protein PER2 through binding within its AUUUU/A motif in RNA [43]. This finding opens a novel perspective for future investigations into the trans-spatial and temporal regulation of diverse cellular activities via LLPS [44] SGs is conducive to cell survival [45]. In virus-induced stress response, the cell’s protein translation is inhibited. At the same time, eukaryotic initiation factor 2α kinase in SGs can efficiently activate NF-κB and IRF3 on the innate immune signaling pathway, which recruits antiviral proteins and initiates antiviral response [46].
Chromatin organization
Chromatin is a condensed and organized structure composed of histones and DNA. LLPS plays a crucial role in chromatin compartmentalization [47]. Protein purification experiments initially revealed that heterochromatin protein 1 (HP1) possesses the capability to create LLPS droplets, and it will be considerably diminished when lysine is substituted with an uncharged amino acid [48]. Furthermore, HP1 is more prone to form droplets when interacting with single-stranded DNA compared to double-stranded DNA [49]. Another study found that methyl-CPG binding protein 2 can induce LLPS in vitro and compete with histone HP1 to generate chromatin condensates. Besides, the binding of methyl-CPG protein 2 to DNA makes it easier to form chromatin condensates [50, 51].
Signal transduction
Various signaling pathways rely on LLPS, such as RTK, Hippo, JAK-STAT3, and mTOR signaling pathways. The RTK signaling pathway depends on the phosphorylation of a terpolymer complex, which contains fibroblast growth factor receptor 2, the phosphatase SHP2 and PLCγ1. It serves as a scaffold to interact with downstream molecules on the plasma membrane [52, 53]. In the Hippo signaling pathway, the core protein kinase LATS2 forms a condensate and recruits important components to promote signaling activation and the cells’ apoptosis [54].
LLPS exists in the synaptic transmission. The postsynaptic density is a membraneless structure located beneath the postsynaptic plasma membrane and is characterized by proteins in high concentrations, where the cognitively deficient synaptic-associated protein SynGAP interacts with PSD-95 via LLPS [55, 56]. In conclusion, the optimal level of LLPS is necessary for transmission within cellular systems.
LLPS in liver innate immune signaling pathways
cGAS-STING pathway
The cGAS-STING pathway is robust in DNA sensing and innate immune response [57]. Following viral infection and cellular damage, there is a notable increase in DNA concentration within the cytoplasm. Subsequently, a cGAS-dsDNA complex is created into a high-concentration droplet via LLPS, which induces the catalytic domain rearrangement of cGAS, ultimately leading to the synthesis of cyclic GMP-AMP (cAMP). In the resting state, STING is primarily localized in the ER [58]. Upon binding of the cyclic GMP-AMP (cGAMP), it performs a conformational change and transfers to the Golgi apparatus. In fact, the translocation event is accompanied by post-translational modifications and subsequent activation of TBK1. Then, IRF3 recruited by STING will be phosphorylated by TBK1, then translocating into the nucleus as a dimer to regulate the transcription of type I IFN [59, 60]. (Fig. 4) At present, nanoparticle-delivered Svg3 has shown great potential as a cGAS agonist oligonucleotide in cancer combination immunotherapy [61].
Schematic model of cGAS-STING and RIG-I-MAVS signaling pathway via LLPS. After sensing and recognizing DNA from itself or viruses, cGAS will combine with DNA and assemble into a complex. The cGAS-DNA droplets activate cGAMP, which further binds to STING on the endoplasmic reticulum membrane and activates the STING-TBK1-IRF3 axis, eventually inducing type I IFN expression. After DNA viral infection, RIG-I will detect RNA quickly. When binding to the K63 polyubiquitin chain, RIG-I interacts with MAVS through their CARD domain. Subsequently, a fraction of MAVS molecules will undergo initial aggregation via LLPS, and then more MAVS molecules form a large condensate, which triggers the RIG-I signal cascade and causes the activation of NF-κB, resulting in the type I IFN expression. Too high concentration of cGAMP can induce STING to pull ER together and form a cubic membrane-like structure, which hinders downstream signal transduction [62]. Obviously, cells regulate the cGAS-STING pathway through LLPS to maintain the optimal level of innate immunity, which enables cells to effectively counteract external pathogens while safeguarding their own tissues from harm. ATP: adenosine triphosphate; GTP: guanosine triphosphate; cGAS: cyclic GMP-AMP synthase; cGAMP: cyclic GMP-AMP; STING: stimulator of interferon genes; TBK1: TANK-binding kinase 1; IKK: inhibitor of kappa B kinase; IRF3: interferon regulatory factor 3; NF-kB: nuclear factor kappa-B; RIG-I: retinoic acid-inducible gene I protein; MAVS: mitochondrial antiviral signaling protein; CARD: caspase activation and recruitment domains
RIG-I-MAVS pathway
RIG-I is an RNA sensor in the innate immune response. It is expressed not only in immune cells but also in hepatocytes [63]. As an adapter protein in the downstream signal transduction, MAVS interacts with RIG-I by their caspase activation and recruitment domains (CARDs). Additionally, MAVS possesses a short transmembrane domain at its C-terminal region responsible for anchoring itself to the mitochondria membrane [64]. In virus-infected cells, RIG-I detects viral RNA, and upon binding to the K63 polyubiquitin chain, RIG-I combines with MAVS. A fraction of MAVS molecules undergoes initial aggregation via LLPS, followed by subsequent recruitment of more MAVS molecules to form larger condensates. It triggers the RIG-I signal cascade and leads to the activation of NF-κB and IRF3, resulting in the type I IFN gene expression [65]. Therefore, the balance of RIG-I-MAVS is crucial in liver diseases, especially those caused by concomitant viral infections.
Inflammasome pathway
Inflammasome is a polymeric protein complex that triggers inflammation in response to exogenous pathogens or endogenous danger signals. This complex is composed of a sensor (a member of the NLR family), an adaptor protein (apoptosis-associated speck-like protein), and an effector protein (procaspase-1) [66]. In liver disease, the NLR family involved in inflammasome includes NLRP1, NLRP3, NLRP6, NLRC4, and AIM2 [67]. In the canonical inflammasome pathway, the inflammasome promotes the secretion of IL-1β, IL-18, and IL-33, and causes a systemic or local inflammatory response to initiate pyroptosis [68].
Fluorescence recovery after photobleaching has found that dsRNA can induce NLRP6 with multiple repeated lysines to emerge LLPS in vitro. NLRP6-dsRNA droplets are highly dynamic, and a hepatitis virus-infected mice model further confirmed the NLRP6 condensates in vivo [69]. It explains that LLPS acts as a switch to start the inflammatory response so that it integrates various signals and resists the external virus attack in an orderly manner.
Autophagy
Autophagy is an ancient innate immune homeostatic process that clears cytoplasmic protein aggregates or engulfs extracellular debris through mitochondria and lysosomes [70]. Theoretically, autophagy possesses anti-inflammatory functions [71]. It can degrade liquid condensates, and the pre-autophagosomal structure also undergoes LLPS to regulate autophagosome [72]. Studies have shown that LLPS is involved in selective autophagy, where droplets containing p62 and receptor proteins determine the direction of the isolation membrane extension [73]. Droplet-like p62 can recognize polyubiquitin chains on target proteins, which then recruit autophagy-related proteins such as ATG8 to form autophagosomes, thereby promoting autophagic degradation [74]. Clinically, this mechanism has been confirmed to provide protective effects against Huntington protein-induced cell death [75]. p62 is a common component of many disease-associated cellular inclusions, such as Mallory-Denk bodies, which are present in alcoholic hepatitis and alcoholic cirrhosis patients. Therefore, specifically targeting and degrading pathogenic proteins in the liver by controlling linker protein LLPS appears to be a promising and innovative therapeutic strategy.
Research indicates that dysregulation of autophagy is associated with various liver diseases [76]. Intracellular proteins can condense into LLPS droplets under certain physicochemical conditions. These cytotoxic protein aggregates are selectively degraded via autophagy. For instance, autophagy intersects with lipid homeostasis, where lipid droplets may mediate the breakdown through LLPS, thereby providing energy for the liver [77].
Liver innate immunity and diseases
Emerging evidence extends that overactivated innate immunity may contribute to liver disorders, including viral hepatitis, non-viral hepatitis, non-alcoholic fatty liver disease (NAFLD), liver fibrosis, liver ischemia-reperfusion injury (IRI), autoimmune liver disease (ALD), and liver cancer [78]. Therefore, we aim to artificially manipulate the strength of the liver’s innate immunity utilizing the regulatory law of LLPS (Fig. 5) (Fig. 6) (Table 1).
A model of liver diseases with innate immune inflammation as the central link. The liver is an immune organ with abundant innate immune cells including kupffer cells and natural killer cells. Hepatic stellate cells and endothelial cells are also involved in liver innate immunity. This induces inflammation and promotes the production of many inflammatory cytokines, which contribute to various liver diseases as shown in the figure. IFN: Interferon; IRF3: interferon regulatory factor 3; TNF: tumor necrosis factor; ROS: reactive oxygen species
A pattern diagram of LLPS involvement in different liver constituent cells. The liver is an immune organ composed of various cells, including hepatocytes, Kupffer cells, epithelial cells, hepatic stellate cells, and immune cells (e.g. NK cells, T cells, and B cells). Hepatocytes contain abundant LLPS, including cccDNA, p62, NLRP6, glycogen molecules, and MAVS-STING. KCs contain lncRNA MALR-ILF3 and phagocytic protein WIP/WASP droplets. Different cells perform specific physiological functions to maintain homeostasis or impact disease progression driven by LLPS. cGAS: cyclic GMP-AMP synthase; LLPS: liquid-liquid phase separation; NK: natural killer; SG: stress granules; STING: stimulator of interferon genes; TNFα: tumor necrosis factor-α; DDX3X: DEAD-box helicase; cccDNA: covalently closed circular DNA; p62: Sequestosome 1; ATG8: autophagy-related proteins; Keap1: kelch like ECH associated protein 1; Nrf2: nuclear factor-erythroid 2-related factor 2; WASP: Wiskott–Aldrich syndrome protein; WIP: WASP-interacting protein; MALR: mammalian apparent long terminal repeat (LTR) retrotransposons; ILF3: interleukin enhancer binding factor 3; HIF1α: hypoxia-inducible factor 1α; NLRP3: NOD-like receptor family pyrin domain containing 3
Viral hepatitis
Viral hepatitis is an infectious disease caused by multiple hepatitis viruses, the most common being hepatitis B virus (HBV) and hepatitis C virus (HCV). The strength of the antiviral immune response depends on the LLPS in the immune pathway [79, 80]. The cGAS-STING pathway and hepatitis virus infection are interdependent. On the one hand, by recognizing nucleic acid components, cGAS-STING prevents viral replication by initiating downstream inflammatory factors [81]. On the other hand, hepatitis viruses evade the cGAS sensing by employing many tactics. For example, the hepatitis B virus X protein (HBx) directly stimulates the ubiquitination and autophagic degradation of cGAS, which down-regulates the production of type I IFN [82]. HCV disrupts the interaction between STING and TBK1 by NS4B proteases to inhibit RNA-induced IFN activation [83].
RIG-I targeting activation has been confirmed as a new treatment for viral hepatitis. The long-term existence of cccDNA in the hepatocyte nucleus is typical for chronic HBV hepatitis [84]. RIG-I agonists can effectively resist cccDNA by activating immune responses [85]. In a study, the co-delivery of siRNA targeting HBx and IL-12 plasmid to hepatocytes upregulates MAVS and RIG-I, thereby reversing virus-induced immune suppression [86]. Interestingly, cccDNA undergoes LLPS in a G-quadruplex-dependent manner, which accelerates HBV propagation within liver cells. This suggests that disrupting the stability of G4 structures may offer a potential strategy to alleviate chronic infections [87]. In HCV-infected hepatocytes, the expression of RIG-I and MDA5 is significantly decreased compared with other PRRs, which reveals that signal amplification of RIG-I-MAVS may serve as a crucial target for antiviral immunotherapy [88]. It is worth noting that HCV uses innate pathways to exploit its infectious advantage, thereby promoting HCV self-assembly and hepatic lipogenesis [89].
Both HBV and HCV infections impair autophagic flux [90, 91]. The HBx and HCV NS5B enhance the autophagosome formation in liver cells [92]. HBx reduces lysosomal targeting by interacting with V-ATPase, thereby impairing autophagic degradation. On the other hand, HCV defects autophagic flux by disrupting the fusion of autophagosomes with lysosomes [93]. Therefore, autophagy can serve both as an antiviral defense mechanism and as a process that supports viral replication.
Non-viral hepatitis
Non-viral hepatitis refers to a group of hepatitis mainly including steatohepatitis, alcoholic hepatitis, and drug-induced liver injury (DILI) [94]. Continuous and prolonged hepatic cellular oxidative stress and liver inflammatory stimuli are key signatures of DILI. Excessive acetaminophen can promote the production of reactive oxygen species (ROS) and induce mitochondria to release mtDNA, thus activating the cGAS-STING [104]. Hepatocyte DDX3X protects against DILI by controlling SGs formation and oxidative stress [95]. Acetylation of the IDR region in DDX3X is essential for enhancing LLPS propensity and SG maturation. The deacetylase SIRT6 is a critical regulator of SGs [105]. Therefore, specific acetylation modifications are closely associated with liver diseases.
Autophagy can protect against DILI by selectively removing damaged mitochondria and drug conjugates. For example, chlorpromazine alleviates DILI by activating autophagy [106]. Additionally, IL-22 prevents DILI in mice by activating AMPK-dependent autophagy [107]. Therefore, targeted autophagy presents an effective strategy for the prevention of DILI.
NAFLD
NAFLD is the most common chronic liver disease worldwide [108]. For example, a high-fat diet can activate the cGAS-STING response in adipose tissue, further leading to obesity, insulin resistance, and metabolic dysfunction [109]. Immunohistochemistry has shown that STING levels in hepatocytes of NAFLD are higher than those of non-NAFLD. On the contrary, loss of STING in macrophages mitigates hepatic steatosis and reduces serum levels of cholesterol, triglycerides, and low-density lipoproteins [110, 111]. In addition, lipid overload in hepatocytes leads to replication stress and DNA damage, which stimulates specific upregulation of cGAS-STING [112]. These suggest that the inflammatory response and the lipid metabolism disorder are mutually reinforcing.
The dysregulated immunity contributes to the transformation of non-alcoholic fatty liver (NAFL) to non-alcoholic steatohepatitis (NASH) [113]. Immunohistochemical analysis of the human liver has shown that the RIG-I level of advanced NASH is significantly stronger than that of NAFL, besides, the mean IRF3 staining intensity in NASH bile duct is higher than that in NAFL [114]. The RIG-I-MAVS pathway plays a contributing role in the deterioration of NAFLD.
Autophagy serves as a protective mechanism against lipid toxicity in NAFLD. However, hepatic autophagy and lysosomal function are compromised in disease states, leading to more severe pathogenic steatosis [115, 116]. In hepatocytes, p62 and ATG8 form autophagosomes, and subsequently, Keap1 can bind within the p62 gel reversibly, activating the transcription factor Nrf2 [116] These findings suggest that p62 gels may function as a platform for Nrf2-mediated inflammatory responses. Further research is needed to elucidate the role of LLPS-mediated selective autophagy in liver diseases. Additionally, it is worth exploring whether enhancing liver lipid droplet clearance through the ubiquitin-proteasome system via LLPS could be a viable strategy.
PTMs are involved in NAFLD. A study indicates that acetyl-CoA helps limit the autophagic degradation of lipid droplets, acting as a key regulator of hepatic lipid homeostasis [117]. Evidence shows that deacetylase SIRT3 is reduced in NAFLD [118]. Additionally, the phosphorylated insulin-induced gene weakens its interaction with the E3 ubiquitin ligase gp78, thereby inhibiting hepatic lipogenesis [119]. Therefore, inhibiting acetyl-CoA production or modulating specific acetylation/ubiquitination in the liver could be a novel therapeutic option for NAFLD.
Liver fibrosis
In the process of chronic liver disease, excessive deposition and abnormal distribution within the extracellular matrix in the liver will lead to fibrosis. The activated KCs and hepatic stellate cells (HSCs) are the core pathogenesis of hepatic fibrosis [120, 98]. For example, X-box binding protein 1 can promote the mtDNA leakage from KCs and induce innate immunity [121]. Iron death in hepatocytes will cause oxidative DNA damage and stimulate STING in KCs, which establishes an activated immune microenvironment to promote liver injury and fibrosis [99]. Accordingly, the upregulation of cGAS-STING has been reported to aggravate liver inflammation in liver fibrosis patients and mice models [101]. However, activated cGAS-STING can inhibit endothelial cell proliferation, thus showing the potential to alleviate liver fibrosis [102]. To sum up, there is still controversy regarding the aggravating or mitigating effects of the cGAS-STING pathway on liver damage, and whether these effects are dominant in specific situations subject to further consideration.
Autophagy promotes HSC activation. In vitro studies have shown that autophagic activity significantly increases in fibrous mouse or human HSCs [122]. Various signaling pathways can activate autophagy in HSCs to promote fibrosis, such as the Akt/mTOR, ERK, and JNK pathways [116]. Additionally, p62 can directly bind to the vitamin D receptor and retinoid X receptor, urging their heterodimerization and thereby suppressing HSC activation [123]. Therefore, the accumulation of p62 due to autophagy inhibition may inhibit liver fibrosis through LLPS.
Liu ZY demonstrated that phosphorylation of heat shock protein 27 under stress enhances FUS LLPS, maintaining a liquid phase to prevent amyloid fibril formation [124]. Although no specific molecules have been identified that undergo spontaneous LLPS in liver fibrosis, it is possible that PTMs crosstalk-induced protein aggregation changes may occur in the context of complex stress disorders and extensive protein deposition.
IRI
IRI, manifested by innate immune-mediated inflammation and stress-induced oxidative damage, usually occurs after hepatectomy and liver transplantation, leading to liver dysfunction and transplant failure [125]. Hepatocytes release significant mtDNA during IRI, it will be recognized by cGAS-STING and contribute to the production of inflammatory cytokines, thus aggravating IRI [126]. Notably, cGAS-mediated autophagy can protect the liver in a STING-independent manner [127]. So, the localization pattern of STING in different cells, and the tissue specificity of cGAS-STING pathway both remain to be explored.
Previous studies have found that ROS secreted by IRI contributes to the MAVS activation and further LLPS aggregation in vivo, which finally excites downstream inflammatory pathways [128]. Paradoxically, new experiments demonstrated that reduced MAVS expression can enhance apoptosis and mitophagy in IRI, accompanied by higher plasma alanine aminotransferase and tumor necrosis factor-α [129]. It indicates a protective role for MAVS in liver inflammation.
ALD
ALD is a special chronic liver disease caused by immune dysfunction, including autoimmune hepatitis (AIH), primary biliary cholangitis, and primary sclerosing cholangitis (PSC). Droplets formed by cGAS and DNA inhibit the nuclease TREX1, this limits self-DNA degradation and leads to immune overactivation, finally resulting in AIH [130] In the AIH mice model, overload manganese establishes a hepatic inflammatory microenvironment and aggravates liver injury by activating the cGAS-STING pathway [131]. Drugs targeting the LLPS of cGAS-DNA may offer a valid treatment for AIH and other autoimmune diseases. However, the cGAS-STING pathway in other ALDs is rarely reported.
The dual role of NLRP3 inflammasomes in PSC has been confirmed. On the one hand, by releasing inflammatory factors to increase liver injury and fibrosis. For example, studies have shown that galectin 3 can activate NLRP3 inflammasome in KCs and HSCs to drive primary biliary cholangitis [103]. On the other hand, blocking other cell death pathways protects the PSC during acute cholestasis liver damage [132]. It can be seen that the innate immune and the cell death pathways interact to affect the balance of liver disease states.
Liver cancer
Liver cancer is a primary malignant tumor derived by persistent chronic liver damage, inflammation, and compensatory hyperplasia [133]. cGAS-STING recruits immune cells to gather and clear cancer cells by enhancing their susceptibility to immune attack by NK cells and CTLs [134]. Currently, STING agonists are used as adjuvants in cancer vaccines by activating anti-tumor immunity [12]. Interestingly, the activation of the intrinsic cGAS-STING pathway in cancer cells also mediates immune cloaking after radiation therapy-induced DNA damage [135].
We consider RIG-I as a tumor suppressor, and overloaded RIG-I expression will be against proliferation, migration, and invasion of cancer cells [136]. A study found that in the diethylnitrosamine-induced HCC development model, the demethylase JMJD4 can demethylate RIG-I and prevent the IL-6-STAT3 signaling pathway to impair anti-tumor immunity. In other words, reduced RIG-I in carcinoma progenitor cells drives progression from HcPC to HCC [137]. It should be pointed out that RIG-I binds with STAT to amplify the IFN reaction in the above study [63]. Therefore, whether RIG-I has crosstalk and additional effects between the two pathways in viral HCC still needs to be verified.
Autophagy inhibits tumors, and defects in autophagy can promote liver cancer. The activation of oncogenes or loss of tumor suppressor genes can lead to mTORC1 activation [138]. Dysregulation of the p62-Keap1-Nrf2 axis has been observed in human and mouse HCC. The increased Keap1-p62 aggregates are associated with improved liver function [96]. Therefore, accelerating early autophagosome formation and autophagic protein aggregation through LLPS could be a potential therapeutic approach to liver cancer.
PTMs play a crucial role in cancer progression. N6-methyladenosine exerts biological effects by dynamically regulating methylation levels, including immunity, tumorigenesis, and adipogenesis [139]. The coiled-coil (CC) domain of the ubiquitin ligase RNF214 promotes the growth and migration of HCC through LLPS [140]. Additionally, SIRT1 is overexpressed in HCC, it promotes HCC in an obesity-dependent manner and involves crosstalk with mRNA methylation, protein acetylation, and ubiquitination [141]. Thus, PTMs of proteins or RNA influence their biomolecular aggregation through LLPS and may serve as biomarkers for early diagnosis of liver cancer.
Biomolecular condensates in liver cancer
LLPS enhances oncogenic signaling pathways and advances cancer progression, and has been proposed as a promising cancer biomarker and intervention target. In addition to the above-mentioned pathways, biomolecular condensates formed by other molecules are present in liver cancer [100].
Crafty tumors take advantage of LLPS as a strategy to evade innate immunity. For example, the cells’ growth needs more glucose consumption, when LLPS occurs in sufficient glycogen, the Laforin-Mst1/2 complex is wrapped together, which jointly activates the oncoprotein Yap, leading to liver growth and tumor transformation [97]. Recent evidence revealed that the acetyltransferase KAT8 and IRF1 will form a droplet and localize to the PD-L1 promoter, prompting the transcription and expression of PD-L1 [142]. In addition, the fusion oncoprotein PKA, which is linked to atypical liver cancer, can effectively block cAMP LLPS and result in abnormal signaling [143]. There are many other mutant proteins that improve the survival advantage and chemotherapy resistance of tumor cells by disrupting LLPS, such as tumor suppressor factors SPOP, [144]. PTEN, [145]. USP42 [146], . and SHP2 [15].
LLPS has also been observed to exert an anti-tumor effect. RNA interference technology demonstrated that circVAMP3 acts as a molecular scaffold by facilitating the LLPS assembly of SGs with CAPRIN1. This subsequently suppresses cancerous proliferation and migration [147].
Anti-tumor therapy focused on LLPS
Abnormal LLPS are regarded as carcinogenic factors. At present, studies have confirmed that LLPS is involved in a variety of cancers by condensing oncoproteins and changing chromatin structure, such as liver cancer, lung cancer, kidney cancer, ovarian cancer, leukemia, etc. [148]. Therefore, this section discusses anti-tumor efforts focused on the whole cancers, not limited to HCC [149]. The application of single-molecule magnetic resonance, atomic force microscopy, and freezing electron microscopy, along with the LLPS protein database PhaSepDB have provided us with great convenience to deepen the understanding of LLPS [150, 151].
The fusion protein is a prevalent mechanism that LLPS contributes to cancer progression. Scientists developed a high-throughput screening technique known as DropScan to find compounds that can regulate abnormal LLPS condensates. It offers a wide possibility for cancer treatment by targeting condensate fusion protein [152]. Recently, a series of chemical probes have been developed that exhibit binding affinity sensitive to the LLPS microstructure, enabling visualization, quantitative analysis, and even manipulation [153].
Certain antitumor medications also can engage in agglomerate assembly. Wang et al. have revealed that adriamycin exhibits specific binding to histone HP1. It induces an overall conformational change via LLPS, which suggests the latent role of LLPS in boosting drug enrichment at specific sites [154].
Concluding remarks and future perspectives
In a word, LLPS provides a new perspective on innate immune-mediated liver diseases. Our study comprehensively elaborates on the innate immune signaling pathways and pathological mechanisms of various liver diseases based on LLPS. Furthermore, we highlight the potential significance of LLPS in anti-tumor immunity and therapy techniques targeting it.
From viral hepatitis or NAFLD to liver fibrosis, and further to cirrhosis and cancer, it is worth noting that the immune signaling runs through the whole process of liver diseases. Under healthy conditions, hepatic immune cells maintain tolerance to circulating antigens and endotoxins. In response to external stimuli, the liver can rapidly induce the accumulation and infiltration of immune cells, while simultaneously triggering autoimmune inflammation which in turn leads to organ damage [155].
Cells infected with hepatitis viruses are primarily exposed to persistent signaling mediated by NK cells, NKT cells, and KCs, leading to chronic liver inflammation. In NAFLD, excessive lipotoxicity continuously assaults innate immune cells, promoting persistent TLR signaling. Under oxidative stress, KCs differentiate into pro-inflammatory macrophages, producing large amounts of interleukins (IL-1β, IL-12, IL-23), leading to NASH, liver damage, and insulin resistance [156]. In alcoholic hepatitis, ethanol overload drives KCs and CD4 + Th cells to secrete large amounts of IL-17, which collectively sustains liver inflammation [157]. Subsequently, the liver immune microenvironment stimulates HSCs to transform into a proliferative and fibrogenic phenotype, upregulating collagen synthesis activity and resulting in liver fibrosis [155]. This progressively worsens parenchymal damage, potentially advancing to late-stage cirrhosis and liver cancer. LLPS has been shown to be involved in immune signaling pathways and autophagy in various liver constituent cells. Consequently, immunotherapy, particularly immune checkpoint inhibitors, shows promise in the management of liver diseases [158]. In HCC, activated RIG-I is necessary for immune checkpoint blockade to exert anti-tumor efficacy, which poses a higher challenge for us to balance immunity as a therapeutic target [159]. Therefore, a better understanding of LLPS-related immune dysregulation is essential to advance precision immunotherapy for liver disease.
It is predicted that more than 30% of the human proteome tends to form LLPS condensates [160]. We should pay attention to the altered physicochemical properties of LLPS in liver diseases. For example, biomolecular condensation can be affected by the stiffness of the surrounding matrix. In liver fibrosis, excessive deposition of diffuse extracellular matrix will lead to increased hardness, which may alter the agglomerates assembly. Abnormal glycolipid metabolism or cellular damage may also affect LLPS by changing pH and osmotic pressure in the liver [161].
We hope to improve the disease state by regulating the local strength of liver immunity through LLPS. However, it is important to acknowledge that our research has certain limitations inevitably. First, the relevant literature was indeed limited about the involvement of LLPS itself in liver diseases, and we did our utmost to utilize the available resources to support our conclusions. We will continue to explore future research to enhance the comprehensiveness and scientific rigor of our study. Additionally, many experiments are only conducted in vitro or in animal models, which limits the application to clinical treatment [151, 153]. Even though several drugs have been designed to disrupt condensates, their suitability still requires further verification. Furthermore, the strategies for preventing viral and tumor cells’ off-target still need more investigations.
Finally, we still hope that scientists will continue to contribute valuable insights and make significant advancements in clinical medicine, thereby paving the way for a new pattern on LLPS.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- ALD:
-
Autoimmune liver disease
- AIH:
-
Autoimmune hepatitis
- CARD:
-
Caspase activation and recruitment domain
- cAMP:
-
Cyclic AMP
- cGAMP:
-
Cyclic GMP-AMP
- cGAS:
-
Cyclic GMP-AMP synthase
- DILI:
-
Drug-induced liver injury
- ER:
-
Endoplasmic reticulum
- FUS:
-
Fused in sarcoma
- G3BP1:
-
GAP SH3 binding protein 1
- HBV:
-
Hepatitis B virus
- HBx:
-
Hepatitis B virus X protein
- HCC:
-
Hepatocellular carcinoma
- HSCs:
-
Hepatic stellate cells
- HCV:
-
Hepatitis C virus
- IDR:
-
Intrinsically disordered region
- IFN:
-
Interferon
- IRF3:
-
Interferon regulatory factor 3
- IRI:
-
Ischemia-reperfusion injury
- IL:
-
Interleukins
- KC:
-
Kupffer cells
- LCD:
-
Low complexity domain
- LLPS:
-
Liquid-liquid phase separation
- MAVS:
-
Mitochondrial antiviral signaling protein
- NAFL:
-
Non-alcoholic fatty liver
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- NLRs:
-
NOD-like receptors
- NK:
-
Natural killer
- PKA:
-
Protein kinase A
- PRRs:
-
Pattern recognition receptors
- PTM:
-
Post-translational modification
- RIG-I:
-
Retinoic acid-inducible gene I protein
- ROS:
-
Reactive oxygen species
- SG:
-
Stress granule
- STING:
-
Stimulator of interferon genes
- TBK1:
-
TANK-binding kinase 1
- TNFα:
-
Tumor necrosis factor-α
References
Banani SF, et al. Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol. 2017;18(5):285–98.
Wang B, et al. Liquid-liquid phase separation in human health and diseases. Signal Transduct Target Ther. 2021;6(1):290.
Niu X, et al. Biomolecular condensates: formation mechanisms, biological functions, and therapeutic targets. MedComm. 2020;2023(42):pe223.
Alberti S, Dormann D. Liquid-liquid phase separation in Disease. Annu Rev Genet. 2019;53:171–94.
Liu-Yesucevitz L, et al. Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue. PLoS ONE. 2010;5(10):e13250.
Zhang L, et al. Phase separation in kidney diseases: autosomal Dominant polycystic kidney Disease and Beyond. Kidney Dis (Basel). 2023;9(4):229–38.
Wang X, et al. Development of cyclopeptide inhibitors of cGAS targeting protein-DNA interaction and phase separation. Nat Commun. 2023;14(1):6132.
Liu D, Yang J, Cristea IM. Liquid-liquid phase separation in innate immunity. Trends Immunol; 2024.
Neshat SY et al. Liver Disease: induction, progression, immunological mechanisms, and therapeutic interventions. Int J Mol Sci, 2021. 22(13).
Li J, et al. Macrophage mitochondrial fission improves cancer cell phagocytosis induced by therapeutic antibodies and is impaired by glutamine competition. Nat Cancer. 2022;3(4):453–70.
Chen H, et al. Self-programmed dynamics of T cell receptor condensation. Proc Natl Acad Sci U S A. 2023;120(28):e2217301120.
Chen B, et al. cGAS-STING Signaling Pathway and Liver Disease: from Basic Research to Clinical Practice. Front Pharmacol. 2021;12:719644.
Hopfner KP, Hornung V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat Rev Mol Cell Biol. 2020;21(9):501–21.
Brangwynne CP, et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324(5935):1729–32.
Li P, et al. Phase transitions in the assembly of multivalent signalling proteins. Nature. 2012;483(7389):336–40.
Kato M, et al. Redox State Controls phase separation of the yeast Ataxin-2 protein via reversible oxidation of its methionine-rich low-complexity domain. Cell. 2019;177(3):711–e7218.
Dundr M, Misteli T. Functional architecture in the cell nucleus. Biochem J. 2001;356(Pt 2):297–310.
Brangwynne CP, Mitchison TJ, Hyman AA. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 2011;108(11):4334–9.
Lafontaine DLJ, et al. The nucleolus as a multiphase liquid condensate. Nat Rev Mol Cell Biol. 2021;22(3):165–82.
Alston JJ, Soranno A. Condensation goes viral: a polymer physics perspective. J Mol Biol. 2023;435(16):167988.
Hyman AA, Weber CA, Jülicher F. Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol. 2014;30:39–58.
Gomes E, Shorter J. The molecular language of membraneless organelles. J Biol Chem. 2019;294(18):7115–27.
Vernon RM et al. Pi-Pi contacts are an overlooked protein feature relevant to phase separation. Elife, 2018. 7.
Nott TJ, et al. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol Cell. 2015;57(5):936–47.
Boeynaems S, et al. Phase separation of C9orf72 dipeptide repeats perturbs stress Granule Dynamics. Mol Cell. 2017;65(6):1044–e10555.
Murray DT, et al. Structure of FUS protein fibrils and its relevance to self-assembly and phase separation of low-complexity domains. Cell. 2017;171(3):615–e62716.
Lubkowska A et al. Role of heat shock proteins (HSP70 and HSP90) in viral infection. Int J Mol Sci, 2021. 22(17).
Peng L, Li EM, Xu LY. From start to end: phase separation and transcriptional regulation. Biochim Biophys Acta Gene Regul Mech. 2020;1863(12):194641.
Zhao YG, Zhang H. Phase separation in membrane Biology: the interplay between membrane-bound organelles and Membraneless Condensates. Dev Cell. 2020;55(1):30–44.
Kwon I, et al. Phosphorylation-regulated binding of RNA polymerase II to fibrous polymers of low-complexity domains. Cell. 2013;155(5):1049–60.
Sang D, et al. Condensed-phase signaling can expand kinase specificity and respond to macromolecular crowding. Mol Cell. 2022;82(19):3693–e371110.
Jiang N, et al. Acetylation in pathogenesis: revealing emerging mechanisms and therapeutic prospects. Biomed Pharmacother. 2023;167:115519.
Liu Y, et al. Crosstalk between protein post-translational modifications and phase separation. Cell Commun Signal. 2024;22(1):110.
Protter DSW, Parker R. Principles and properties of stress granules. Trends Cell Biol. 2016;26(9):668–79.
Sidibé H, Dubinski A, Vande C, Velde. The multi-functional RNA-binding protein G3BP1 and its potential implication in neurodegenerative disease. J Neurochem. 2021;157(4):944–62.
Jin G, et al. G3BP2: structure and function. Pharmacol Res. 2022;186:106548.
Kedersha N, et al. G3BP-Caprin1-USP10 complexes mediate stress granule condensation and associate with 40S subunits. J Cell Biol. 2016;212(7):845–60.
Shakya A, et al. Liquid-liquid phase separation of Histone Proteins in cells: role in chromatin Organization. Biophys J. 2020;118(3):753–64.
Li P et al. High-throughput and proteome-wide discovery of endogenous biomolecular condensates. Nat Chem, 2024.
Li W, Jiang H. Nuclear protein condensates and their properties in Regulation of Gene expression. J Mol Biol. 2022;434(1):167151.
Shrinivas K, et al. Enhancer features that drive formation of Transcriptional Condensates. Mol Cell. 2019;75(3):549–e5617.
Hnisz D, et al. A phase separation model for Transcriptional Control. Cell. 2017;169(1):13–23.
Yokoshi M, et al. Direct binding of Ataxin-2 to distinct elements in 3’ UTRs promotes mRNA stability and protein expression. Mol Cell. 2014;55(2):186–98.
Zhuang Y, et al. Circadian clocks are modulated by compartmentalized oscillating translation. Cell. 2023;186(15):3245–e326023.
Bhatter N, Dmitriev SE, Ivanov P. Cell death or survival: insights into the role of mRNA translational control. Semin Cell Dev Biol. 2024;154(Pt B):138–54.
Eiermann N et al. Dance with the Devil: stress granules and signaling in antiviral responses. Viruses, 2020. 12(9).
Li J, et al. Protein phase separation and its role in chromatin organization and diseases. Biomed Pharmacother. 2021;138:111520.
Strom AR, et al. Phase separation drives heterochromatin domain formation. Nature. 2017;547(7662):241–5.
Leicher R, et al. Single-stranded nucleic acid binding and coacervation by linker histone H1. Nat Struct Mol Biol. 2022;29(5):463–71.
Wang L, et al. Rett syndrome-causing mutations compromise MeCP2-mediated liquid-liquid phase separation of chromatin. Cell Res. 2020;30(5):393–407.
Zhang H, et al. MeCP2-induced heterochromatin organization is driven by oligomerization-based liquid-liquid phase separation and restricted by DNA methylation. Nucleus. 2022;13(1):1–34.
Lin CC, et al. Receptor tyrosine kinases regulate signal transduction through a liquid-liquid phase separated state. Mol Cell. 2022;82(6):1089–e110612.
Ladbury JE, Lin CC, Suen KM. Phase separation enhances probability of receptor signalling and drug targeting. Trends Biochem Sci. 2023;48(5):428–36.
Qin M et al. LATS2 condensates organize signalosomes for Hippo pathway signal transduction. Nat Chem Biol, 2024.
Zeng M, et al. Phase transition in postsynaptic densities underlies formation of synaptic complexes and synaptic plasticity. Cell. 2016;166(5):1163–e117512.
Lv P, et al. O-GlcNAcylation modulates liquid-liquid phase separation of SynGAP/PSD-95. Nat Chem. 2022;14(7):831–40.
Liu C, et al. Phase separation in cGAS-STING signaling: cytosolic DNA sensing and Regulatory functions. ChemBioChem. 2023;24(10):e202300147.
Srikanth S, et al. The ca(2+) sensor STIM1 regulates the type I interferon response by retaining the signaling adaptor STING at the endoplasmic reticulum. Nat Immunol. 2019;20(2):152–62.
Liu S, et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science. 2015;347(6227):aaa2630.
Zhang C, et al. Structural basis of STING binding with and phosphorylation by TBK1. Nature. 2019;567(7748):394–8.
Zhou S, et al. Engineering cGAS-agonistic oligonucleotides as therapeutics for cancer immunotherapy. Mol Ther Nucleic Acids. 2024;35(1):102126.
Yu X, et al. The STING phase-separator suppresses innate immune signalling. Nat Cell Biol. 2021;23(4):330–40.
Hou J, et al. Hepatic RIG-I predicts survival and interferon-α therapeutic response in hepatocellular carcinoma. Cancer Cell. 2014;25(1):49–63.
Seth RB, et al. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122(5):669–82.
Zeng W, et al. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell. 2010;141(2):315–30.
Malik A, Kanneganti TD. Inflammasome activation and assembly at a glance. J Cell Sci. 2017;130(23):3955–63.
Luan J, Ju D. Inflammasome: a double-edged Sword in Liver diseases. Front Immunol. 2018;9:2201.
Mathur A, Hayward JA, Man SM. Molecular mechanisms of inflammasome signaling. J Leukoc Biol. 2018;103(2):233–57.
Shen C, et al. Phase separation drives RNA virus-induced activation of the NLRP6 inflammasome. Cell. 2021;184(23):5759–e577420.
Deretic V, Levine B. Autophagy balances inflammation in innate immunity. Autophagy. 2018;14(2):243–51.
Deretic V. Autophagy in inflammation, infection, and immunometabolism. Immunity. 2021;54(3):437–53.
Fujioka Y, et al. Phase separation organizes the site of autophagosome formation. Nature. 2020;578(7794):301–5.
Agudo-Canalejo J, et al. Wetting regulates autophagy of phase-separated compartments and the cytosol. Nature. 2021;591(7848):142–6.
Sun D, et al. Polyubiquitin chain-induced p62 phase separation drives autophagic cargo segregation. Cell Res. 2018;28(4):405–15.
Bjørkøy G, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171(4):603–14.
Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3–12.
Filali-Mouncef Y, et al. The ménage à Trois of autophagy, lipid droplets and liver disease. Autophagy. 2022;18(1):50–72.
Xu D, et al. The cGAS-STING pathway: Novel perspectives in Liver diseases. Front Immunol. 2021;12:682736.
Guo F et al. Activation of Stimulator of Interferon genes in Hepatocytes suppresses the replication of Hepatitis B Virus. Antimicrob Agents Chemother, 2017. 61(10).
Li M, et al. Kupffer cells support Hepatitis B virus-mediated CD8 + T cell exhaustion via Hepatitis B Core Antigen-TLR2 interactions in mice. J Immunol. 2015;195(7):3100–9.
Li Y, et al. STING signaling activation inhibits HBV replication and attenuates the severity of liver injury and HBV-induced fibrosis. Cell Mol Immunol. 2022;19(1):92–107.
Ding Q, et al. Hepatitis C virus NS4B blocks the interaction of STING and TBK1 to evade host innate immunity. J Hepatol. 2013;59(1):52–8.
He J, et al. Inhibition of hepatitis B virus replication by activation of the cGAS-STING pathway. J Gen Virol. 2016;97(12):3368–78.
Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015;64(12):1972–84.
Lee S, et al. Suppression of hepatitis B virus through therapeutic activation of RIG-I and IRF3 signaling in hepatocytes. iScience. 2021;24(1):101969.
Mu Y et al. Codelivery of HBx-siRNA and plasmid encoding IL-12 for inhibition of Hepatitis B Virus and Reactivation of antiviral immunity. Pharmaceutics, 2022. 14(7).
Giraud G, et al. G-quadruplexes control hepatitis B virus replication by promoting cccDNA transcription and phase separation in hepatocytes. Nucleic Acids Res. 2024;52(5):2290–305.
Vilasco M, et al. The protein kinase IKKepsilon can inhibit HCV expression independently of IFN and its own expression is downregulated in HCV-infected livers. Hepatology. 2006;44(6):1635–47.
Lowey B et al. Hepatitis C virus infection induces hepatic expression of NF-κB-Inducing kinase and Lipogenesis by downregulating miR-122. mBio, 2019. 10(4).
Sir D, et al. Induction of incomplete autophagic response by hepatitis C virus via the unfolded protein response. Hepatology. 2008;48(4):1054–61.
Sir D, Ann DK, Ou JH. Autophagy by hepatitis B virus and for hepatitis B virus. Autophagy. 2010;6(4):548–9.
Khan M, Imam H, Siddiqui A. Subversion of cellular autophagy during virus infection: insights from hepatitis B and hepatitis C viruses. Liver Res. 2018;2(3):146–56.
Liu B, et al. Hepatitis B virus X protein inhibits autophagic degradation by impairing lysosomal maturation. Autophagy. 2014;10(3):416–30.
Petrasek J, et al. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc Natl Acad Sci U S A. 2013;110(41):16544–9.
Luo T, et al. Hepatocyte DDX3X protects against drug-induced acute liver injury via controlling stress granule formation and oxidative stress. Cell Death Dis. 2023;14(7):400.
Bartolini D, et al. Nrf2-p62 autophagy pathway and its response to oxidative stress in hepatocellular carcinoma. Transl Res. 2018;193:54–71.
Liu Q, et al. Glycogen accumulation and phase separation drives liver tumor initiation. Cell. 2021;184(22):5559–e557619.
Wang X, et al. STING expression in monocyte-derived macrophages is associated with the progression of liver inflammation and fibrosis in patients with nonalcoholic fatty liver disease. Lab Invest. 2020;100(4):542–52.
Su W, et al. TAK1 deficiency promotes liver injury and tumorigenesis via ferroptosis and macrophage cGAS-STING signalling. JHEP Rep. 2023;5(5):100695.
Liu J, et al. The Macrophage-Associated LncRNA MALR facilitates ILF3 liquid-liquid phase separation to promote HIF1α signaling in Esophageal Cancer. Cancer Res. 2023;83(9):1476–89.
Luo S, et al. Activation of cGAS-STING signaling pathway promotes liver fibrosis and hepatic sinusoidal microthrombosis. Int Immunopharmacol. 2023;125Pt B:p111132.
Wang L, et al. Pharmacological targeting of cGAS/STING-YAP axis suppresses pathological angiogenesis and ameliorates organ fibrosis. Eur J Pharmacol. 2022;932:175241.
Fernández-Martín JC et al. Gal3 plays a deleterious role in a mouse model of Endotoxemia. Int J Mol Sci, 2022. 23(3).
Dong K, et al. Mixed micelles loaded with hesperidin protect against acetaminophen induced acute liver injury by inhibiting the mtDNA-cGAS-STING pathway. Colloids Surf B Biointerfaces. 2023;233:113656.
Saito M, et al. Acetylation of intrinsically disordered regions regulates phase separation. Nat Chem Biol. 2019;15(1):51–61.
Li Y, et al. Chlorpromazine protects against acetaminophen-induced liver injury in mice by modulating autophagy and c-Jun N-terminal kinase activation. Liver Res. 2019;3(1):65–74.
Mo R, et al. Enhanced autophagy contributes to protective effects of IL-22 against acetaminophen-induced liver injury. Theranostics. 2018;8(15):4170–80.
Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397(10290):2212–24.
Mao Y, et al. STING-IRF3 triggers endothelial inflammation in response to free fatty acid-Induced mitochondrial damage in Diet-Induced obesity. Arterioscler Thromb Vasc Biol. 2017;37(5):920–9.
Yu Y, et al. STING-mediated inflammation in Kupffer cells contributes to progression of nonalcoholic steatohepatitis. J Clin Invest. 2019;129(2):546–55.
Luo X et al. Expression of STING is increased in liver tissues from patients with NAFLD and promotes macrophage-mediated hepatic inflammation and fibrosis in mice. Gastroenterology, 2018. 155(6): p. 1971–1984.e4.
Donne R, et al. Replication stress triggered by nucleotide pool imbalance drives DNA damage and cGAS-STING pathway activation in NAFLD. Dev Cell. 2022;57(14):1728–e17416.
Parthasarathy G, Revelo X, Malhi H. Pathogenesis of nonalcoholic steatohepatitis: an overview. Hepatol Commun. 2020;4(4):478–92.
Schwertheim S et al. Higher pNRF2, SOCS3, IRF3, and RIG1 tissue protein expression in NASH patients versus NAFL patients: pNRF2 expression is concomitantly Associated with elevated fasting glucose levels. J Pers Med, 2023. 13(7).
Nguyen TB, et al. DGAT1-Dependent lipid Droplet Biogenesis protects mitochondrial function during Starvation-Induced Autophagy. Dev Cell. 2017;42(1):9–e215.
Qian H, et al. Autophagy in liver diseases: a review. Mol Aspects Med. 2021;82:100973.
He A, et al. Acetyl-CoA derived from hepatic peroxisomal β-Oxidation inhibits autophagy and promotes steatosis via mTORC1 activation. Mol Cell. 2020;79(1):30–e424.
Fritz KS, et al. Mitochondrial acetylome analysis in a mouse model of alcohol-induced liver injury utilizing SIRT3 knockout mice. J Proteome Res. 2012;11(3):1633–43.
Han Y, et al. Post-translational regulation of lipogenesis via AMPK-dependent phosphorylation of insulin-induced gene. Nat Commun. 2019;10(1):623.
Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60(5):1090–6.
Wang Q, et al. XBP1-mediated activation of the STING signalling pathway in macrophages contributes to liver fibrosis progression. JHEP Rep. 2022;4(11):100555.
Hernández-Gea V, et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology. 2012;142(4):938–46.
Kim KM, et al. Gα(12) overexpression induced by miR-16 dysregulation contributes to liver fibrosis by promoting autophagy in hepatic stellate cells. J Hepatol. 2018;68(3):493–504.
Liu Z, et al. Hsp27 chaperones FUS phase separation under the modulation of stress-induced phosphorylation. Nat Struct Mol Biol. 2020;27(4):363–72.
Ito T, et al. Ischemia-reperfusion injury and its relationship with early allograft dysfunction in liver transplant patients. Am J Transpl. 2021;21(2):614–25.
Jiao J, et al. Expression of STING is increased in monocyte-derived macrophages and contributes to liver inflammation in hepatic ischemia-reperfusion Injury. Am J Pathol. 2022;192(12):1745–62.
Lei Z, et al. cGAS-mediated autophagy protects the liver from ischemia-reperfusion injury independently of STING. Am J Physiol Gastrointest Liver Physiol. 2018;314(6):G655–67.
Ailenberg M, et al. Dynasore enhances the formation of mitochondrial antiviral signalling aggregates and endocytosis-independent NF-κB activation. Br J Pharmacol. 2015;172(15):3748–63.
Ailenberg M, et al. ACTIVATION OF THE MITOCHONDRIAL ANTIVIRAL SIGNALING PROTEIN (MAVS) FOLLOWING LIVER ISCHEMIA/REPERFUSION AND ITS EFFECT ON INFLAMMATION AND INJURY. Shock. 2022;58(1):78–89.
Zhou W, et al. cGAS phase separation inhibits TREX1-mediated DNA degradation and enhances cytosolic DNA sensing. Mol Cell. 2021;81(4):739–e7557.
Saimaier K et al. Manganese Exacerbates ConA-Induced Liver Inflammation via the cGAS-STING Signaling Pathway Inflammation, 2023.
Frissen M, et al. Bidirectional role of NLRP3 during Acute and Chronic Cholestatic Liver Injury. Hepatology. 2021;73(5):1836–54.
Ringelhan M, et al. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19(3):222–32.
Chen R, et al. The role of cGAS-STING signalling in liver diseases. JHEP Rep. 2021;3(5):100324.
Du SS, et al. Radiation Therapy promotes Hepatocellular Carcinoma Immune Cloaking via PD-L1 Upregulation Induced by cGAS-STING activation. Int J Radiat Oncol Biol Phys. 2022;112(5):1243–55.
Liu Z, et al. RIG-I suppresses the migration and invasion of hepatocellular carcinoma cells by regulating MMP9. Int J Oncol. 2015;46(4):1710–20.
Li Z, et al. JMJD4-demethylated RIG-I prevents hepatic steatosis and carcinogenesis. J Hematol Oncol. 2022;15(1):161.
Chao X, et al. Autophagy and liver cancer. Clin Mol Hepatol. 2020;26(4):606–17.
Yang Y, et al. Dynamic transcriptomic m(6)a decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018;28(6):616–24.
He ZJ, et al. Phase separation of RNF214 promotes the progression of hepatocellular carcinoma. Cell Death Dis. 2024;15(7):483.
Liu X, et al. SIRT1 regulates N(6) -Methyladenosine RNA modification in Hepatocarcinogenesis by Inducing RANBP2-Dependent FTO SUMOylation. Hepatology. 2020;72(6):2029–50.
Wu Y, et al. Disrupting the phase separation of KAT8-IRF1 diminishes PD-L1 expression and promotes antitumor immunity. Nat Cancer. 2023;4(3):382–400.
Zhang JZ, et al. Phase separation of a PKA Regulatory Subunit controls cAMP compartmentation and Oncogenic Signaling. Cell. 2020;182(6):1531–e154415.
Bouchard JJ, et al. Cancer mutations of the tumor suppressor SPOP disrupt the formation of active, phase-separated compartments. Mol Cell. 2018;72(1):19–e368.
Boija A, Klein IA, Young RA. Biomol Condensates Cancer Cancer Cell. 2021;39(2):174–92.
Liu S, et al. USP42 drives nuclear speckle mRNA splicing via directing dynamic phase separation to promote tumorigenesis. Cell Death Differ. 2021;28(8):2482–98.
Chen S, et al. circVAMP3 drives CAPRIN1 phase separation and inhibits Hepatocellular Carcinoma by suppressing c-Myc translation. Adv Sci (Weinh). 2022;9(8):e2103817.
Kiang KM, et al. Biomolecular condensates: hubs of Hippo-YAP/TAZ signaling in cancer. Trends Cell Biol. 2024;34(7):566–77.
Chakravarty AK, et al. Biomolecular Condensation: a New Phase in Cancer Research. Cancer Discov. 2022;12(9):2031–43.
Peng Q, et al. Phase separation in Cancer: from the impacts and mechanisms to treatment potentials. Int J Biol Sci. 2022;18(13):5103–22.
Hou C, et al. PhaSepDB in 2022: annotating phase separation-related proteins with droplet states, co-phase separation partners and other experimental information. Nucleic Acids Res. 2023;51(D1):D460–5.
Wang Y, et al. Dissolution of oncofusion transcription factor condensates for cancer therapy. Nat Chem Biol. 2023;19(10):1223–34.
Sun R, et al. Chemical probes for investigating protein liquid-liquid phase separation and aggregation. Curr Opin Chem Biol. 2023;74:102291.
Wang T, et al. Chemical-induced phase transition and global conformational reorganization of chromatin. Nat Commun. 2023;14(1):5556.
Heymann F, Tacke F. Immunology in the liver–from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13(2):88–110.
Tilg H, et al. Non-alcoholic fatty liver disease: the interplay between metabolism, microbes and immunity. Nat Metab. 2021;3(12):1596–607.
Galasso L et al. Inflammatory response in the Pathogenesis and Treatment of Hepatocellular Carcinoma: a double-edged Weapon. Int J Mol Sci, 2024. 25(13).
Pourbagheri-Sigaroodi A, et al. Immune landscape of hepatocellular carcinoma: from dysregulation of the immune responses to the potential immunotherapies. Cell Biochem Funct. 2024;42(5):e4098.
Heidegger S et al. RIG-I activation is critical for responsiveness to checkpoint blockade. Sci Immunol, 2019. 4(39).
Ruff KM, Pappu RV. AlphaFold and implications for intrinsically disordered proteins. J Mol Biol. 2021;433(20):167208.
Jin X, et al. Effects of pH alterations on stress- and aging-induced protein phase separation. Cell Mol Life Sci. 2022;79(7):380.
Acknowledgements
Not applicable.
Funding
The authors sincerely acknowledge the financial support from the National Natural Science Foundation of China (82270633) and the Natural Science Foundation of Hunan Province (2023JJ30932).
Author information
Authors and Affiliations
Contributions
Conceptualization and writing: Xinying Zhang, Ziyue Yang. Figures organization: Xinying Zhang, Huan Li, Chunmeng Fu. Validation and Supervision: Fang Peng, Ning Li, Run Yao. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, X., Yang, Z., Fu, C. et al. Emerging roles of liquid-liquid phase separation in liver innate immunity. Cell Commun Signal 22, 430 (2024). https://doi.org/10.1186/s12964-024-01787-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-024-01787-4